Capacitive deionization (CDI) is a treatment technology we believe holds promise for reducing the levels of total dissolved solids in developing world drinking water sources. Total dissolved solids, or TDS, represents the sum of organic and inorganic molecules and ions, along with suspended colloidal materials. High TDS levels are common in groundwater sources, and limit the utility of those sources. At 500 ppm TDS, there is a noticeable effect the water’s taste; beyond that, it quickly becomes unpalatable.
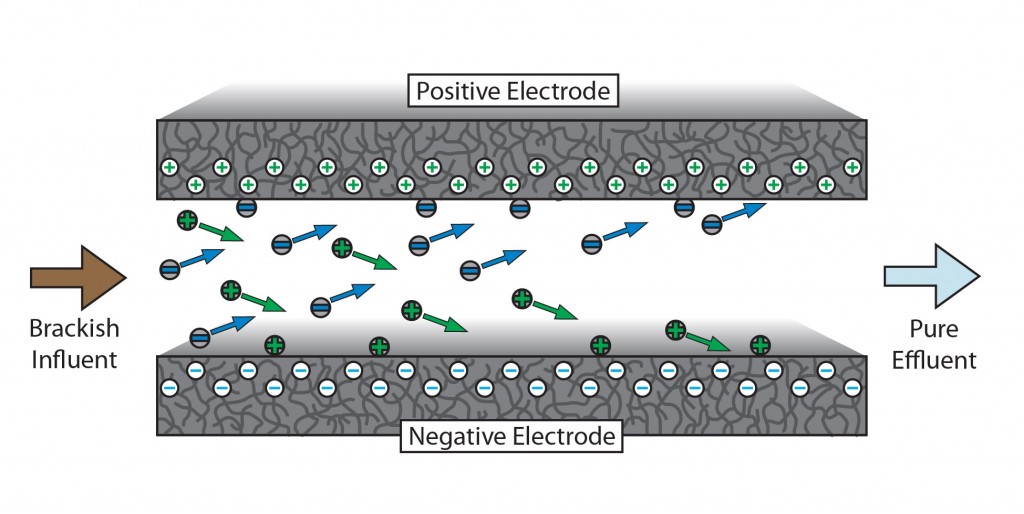
CDI operates by flowing water between two parallel-plate electrodes, each with large surface area. A small voltage is applied to these plates, forming an electric field through the water. Ions in solution drift toward the plates, where they are held in the electrical double-layers at the solid-liquid boundary. The voltages applied are sufficiently low that they do not instigate redox reactions at the electrode surface. When the electrode surfaces become saturated with ions, the flow is paused and the voltages are reversed, driving the ions back into solution. This highly concentrated volume is then diverted to a waste stream, and the cycle begins again.
CDI has been widely explored for desalination of seawater (~35,000 ppm TDS). While it seems unlikely to prove less energy intensive than reverse osmosis in this regime, it compares more favorably as influent concentrations decrease. We are currently looking to treat sources with 1000 to 2000 ppm TDS, and building experiments to gauge efficiency in that regime.
Much of the challenge in CDI lies in the choice of materials used as electrodes. They must be chemically resistant, electrically conductive, physically cohesive, and provide sufficient surface area to entrap large numbers of ions before recycling. While many such materials exist (carbon aerogels, nanostructures), much of the surface area as traditionally measured (via BET, for example), does not contribute substantially to the development of unique double layers and the sorption of ions. Therefore, the specifics of the structure (e.g., pore size distributions) are just as relevant as the total surface area. In developing world applications, material costs also becomes an important factor. Our research is focused on developing efficient, affordable CDI systems by improving ion mobility, and exploring new classes of electrode materials with the potential to increase sorption capacity.
If successful, this technology could be used alone to transform high TDS groundwater sources into viable drinking water supplies. Alternatively, CDI could be paired with treatment technologies for otherwise undetectable groundwater contaminants like arsenic. In such an arrangement, the aesthetic difference between treated and untreated water would then help to increase adoption and to identify untreated supplies.