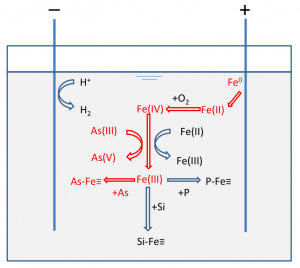
Understanding the chemical kinetics of arsenic during electrocoagulation (EC) treatment is essential for a deeper understanding of arsenic removal using EC under a variety of operating conditions and solution compositions. We describe a highly-constrained, simple chemical dynamic model of As(III) oxidation and As(III,V), Si, and P sorption for the EC system using model parameters extracted from some of our experimental results and previous studies. Our model predictions agree well with both data extracted from previous studies and our observed experimental data over a broad range of operating conditions (charge dosage rate) and solution chemistry (pH, co-occurring ions) without free model parameters. Our model provides insights into why higher pH and lower charge dosage rate (Coulombs/L/min) facilitate As(III) removal by EC, and sheds light on the debate in the recent published literature regarding the mechanism of As(III) oxidation during EC. Our model also provides practically useful estimates of the minimum amount of iron required to remove 500 μg/L As(III) to < 50 μg/L. Parameters measured in this work include the ratio of rate constants for Fe(II) and As(III) reactions with Fe(IV) in synthetic groundwater (k1/k2 = 1.07) and the apparent rate constant of Fe(II) oxidation with dissolved oxygen at pH 7 (kapp = 100.22 M-1s-1).